Abstract
Introduction SC and IV formulations of ALNUC, a 2+1 B-cell maturation antigen (BCMA) x CD3 T-cell engager (TCE) with bivalent binding to BCMA, have been investigated in an open-label, phase 1, first-in-human clinical study in pts with RRMM (NCT03486067). In this study, ALNUC demonstrated promising activity with IV delivery, but elicited CRS in 89% of pts (Costa LJ et al. Blood. 2019;134[Suppl 1]:143). ALNUC recruits T cells to BCMA-expressing myeloma target cells, leading to T-cell cytotoxic activity and cytokine release. Measuring T-cell activating factors and associated cytokine secretion in peripheral blood (PB) during treatment is a useful readout of ALNUC pharmacodynamic (PD) activity and associated CRS. We hypothesized that ALNUC treatment promotes T-cell activity and the secretion of cytokines that are directly correlated with CRS grade, and that SC dosing reduces cytokine secretion compared with IV dosing, thereby improving ALNUC tolerability.
Methods IV and SC ALNUC in the phase 1 study employed step-up dosing (IV schedule: 3 mg starting dose, weekly step-up dosing to 6 mg, and final target dose of 10 mg; SC schedule: 3 mg starting dose at cycle 1 day 1 [C1D1], 3-day step-up dosing to 6 mg [C1D4], and final target doses of 10, 15, or 30 mg). Flat (fixed) doses were explored with IV ALNUC (0.15-10 mg). PB was collected pre- and post-treatment (6, 12, 24, and 48 hrs post administration of the 1st, 2nd, and/or 3rd doses in C1). Plasma analytes in PB samples were measured using Luminex® 17-plex and 8-plex human cytokine arrays. Analyte induction was determined by comparing pre- vs on-treatment concentrations and calculated as fold change.
Results Treatment with IV ALNUC led to dose-dependent and transient release of T-cell specific and pro-/anti-inflammatory cytokines. CRS frequency was highest after the 1st dose (71%; 50 events/70 doses administered) vs 2nd dose (22%; 14 events/63 doses administered), in-line with the blunting of cytokine release typically observed with subsequent infusions (a hallmark of TCE activity). High-grade CRS (grade ≥ 3) was rare in the 70 treated pts but did occur with 3 mg (n = 4) or 6 mg (n = 1) starting doses. Grade ≥ 2 CRS was more common at the 6 and 10 mg starting doses (50%; 6/12 pts) vs the 3 mg starting dose (31%; 17/55 pts), suggesting that higher early exposure is a driving component of CRS in many pts.
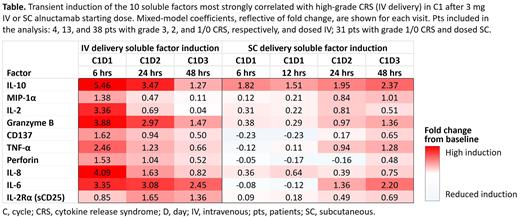
Univariate analyses of soluble factors induced after the 3 mg IV dose identified treatment-induced factors most strongly correlated with high-grade CRS (grade ≥ 3). They included proinflammatory cytokines (IL-10, IL-6, and MIP1α) and factors associated with T-cell activity (IL-2, granzyme B, and TNF-α), the latter highlighting the close relationship between T-cell antitumor activity and CRS. In pts receiving 3 mg SC ALNUC, induction of many of these factors was delayed and reduced (Table). Peak ALNUC serum concentration (Cmax) was also substantially delayed and reduced with the 3 mg SC starting dose vs IV, suggesting that changes in the SC pharmacokinetic profile contributed to the delayed PD effects on soluble factors. Importantly, all grade/grade ≥ 3 CRS was reduced with SC (53%/0%; all regimens; n = 47) vs IV ALNUC (76%/7%; all regimens; n = 70).
Despite lower Cmax with SC vs IV dosing (3 mg starting dose), ALNUC concentrations predicted for efficacy (> 90% maximal effective concentration predicted from nonclinical data) were exceeded at the 30 mg target dose by C2D1 and were comparable to the Cmax of IV ALNUC 10 mg target dose. Notably, a higher SC target dose did not increase CRS severity; all grade/grade 1/grade 2 CRS occurred in 47%/40%/7% of 15 pts who received 3/6/30 mg SC vs 68%/60%/8% of 25 pts who received 3/6/10 mg SC as of the data cutoff (May 31, 2022).
Conclusions IV ALNUC in C1 induced transient and dose-dependent secretion of soluble factors and cytokines associated with T-cell antitumor activity and CRS. IV delivery at higher starting doses promoted strong clinical efficacy in a narrow therapeutic window but also led to severe CRS (grade ≥ 3). SC dosing reduced and delayed the secretion of factors that correlated with high-grade CRS. Plasma exposure to initial SC doses was lower than IV ALNUC; however, the target dose achieved predicted optimal exposure for efficacy. These results suggest that SC formulation widens the therapeutic index of ALNUC, enabling higher target doses to be tested to increase efficacy while reducing release of proinflammatory cytokines and CRS-related toxicity.
Disclosures
Boss:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company. Thompson:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Gaudy:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company. Vu:vumab AG: Current Employment, Membership on an entity's Board of Directors or advisory committees; Celgene EngMab GmbH (BMS): Ended employment in the past 24 months; Roche: Current holder of stock options in a privately-held company; Bristol Myers Squibb: Consultancy. Godwin:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company; Pfizer: Research Funding. Burgess:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months. Wong:Patient Discovery: Research Funding; Caelum: Research Funding; Genentech: Research Funding; GSK: Research Funding; Fortis: Research Funding; Janssen: Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees; Dren Bioscience: Consultancy; Catalent Biologics: Consultancy; Bristol Myers Squibb: Research Funding. Costa:Genentech: Research Funding; AbbVie: Research Funding; Sanofi: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal